Project
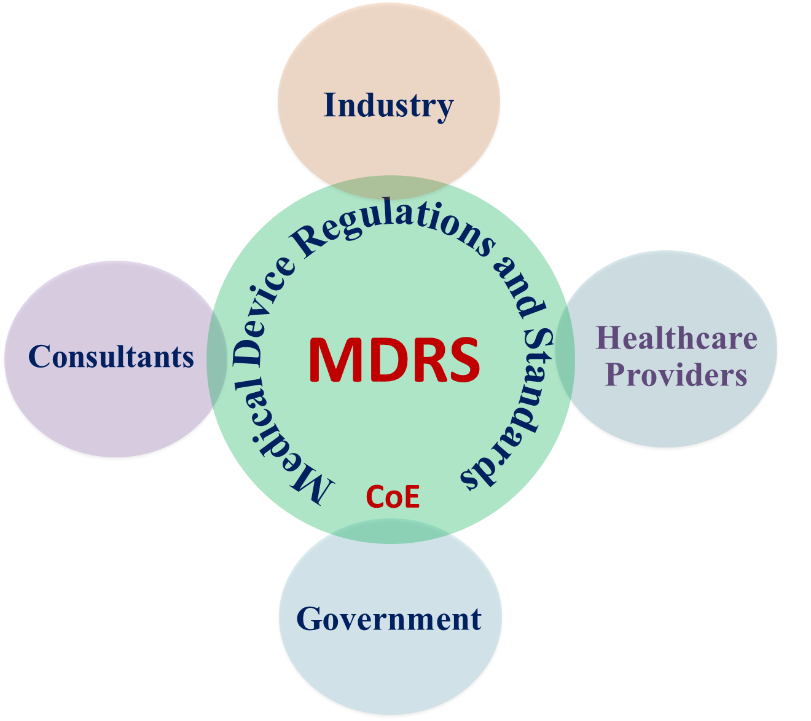
Aim: Establishment of a Centre of Excellence for Medical Device Regulation and Standards at IIT Madras
Functions: Research, generate expertise and help stakeholders to implement global regulations and standards
Key Objectives:
- Facilitate Indian industry to accelerate medical device design and development through comprehensive knowledge on local and global regulations and standards,
- Help manufacturers to understand the regulations, standards and quality system requirements,
- Facilitate regulatory authorities in making informed decision on public health policies related to medical device safety, approvals and monitoring,
- Provide researchers country-specific data and essential training for design, development, verification and validation of medical devices,
- Generate high quality human resource in the areas of medical device design, development, manufacturing, regulatory and hospital auditing, and
- Participate in open innovation under global harmonization efforts
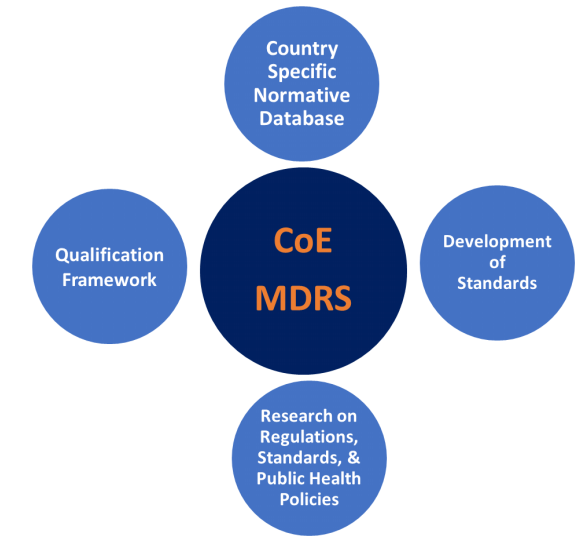
Expected deliverables of the research
- Established strategic partnerships with all healthcare stake-holders in India and abroad, particularly in SMEs, government regulatory authorities, international standard organizations and leading international universities
- Established country specific normative database of Medical Images and Signals, to assist industries in design and validation and to facilitate policy makers to address public health issues
- Demonstrated ability to participate in standards development
- Demonstrated continuous professional development opportunities for quality system health audit experts
- Generation of monographs, publications and resources of standards, regulations and multi-media materials for on-demand multi-modal higher learning materials
- Evolution of independent competent nodal national system for comprehensive regulatory and standard services to medical device industry and practitioners
Current status
Collaborations
International Collaborations
Germany:
Department of Psychiatry, Psychotherapy and Psychosomatics: RWTH Aachen
PLRI institute for Medical Informatics: Technische Universität Braunschweig
Australia:
Melbourne School of Engineering: University of Melbourne
Department of Electrical and Biomedical Engineering: RMIT University
USA:
School of Biomedical Engineering, Science and Health Systems: Drexel University
Department of Neurosurgery: Linda Loma State University
Societal impact
- The regulatory activities of the center would result in enhanced quality of life in elderly through use of reliable and standard self-monitoring devices and wearables.
- Society would be benefit by enhancing their knowledge in wellness pointers which would be shared by the center through various means.
- The regulated technology would fill in the gap between rural-urban divide, and in addition to enhancing the quality of diagnosis through calibrated devices.
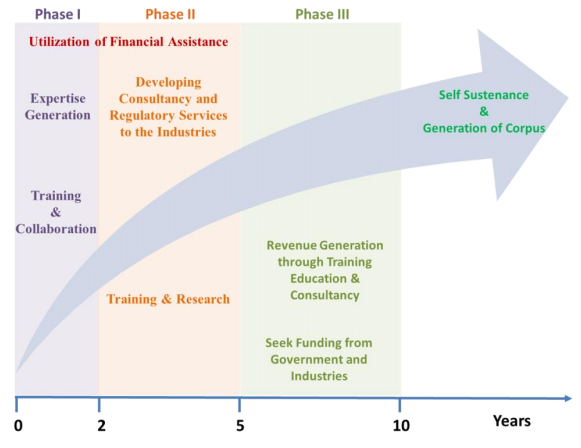
Sustenance statement