Project
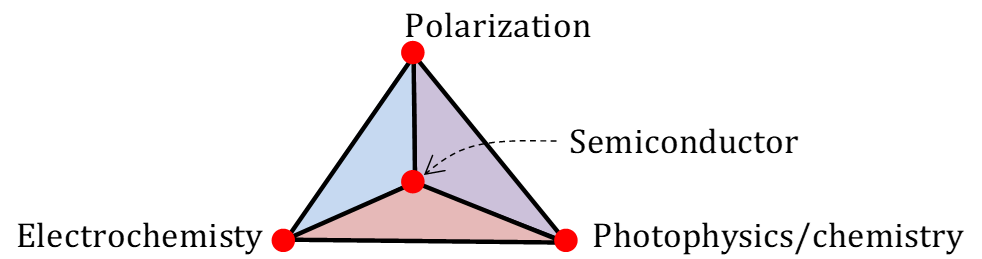
The fundamental issue that is hampering the solar water splitting and the metal-air battery technology from being commercialized is the non-availability of suitably stable and high performing catalyst for the aforementioned two reactions. Conventional approaches to the development of catalysts don’t see the light of the day. So in this project, a ground-breaking idea is developed where in the concept of polarization (dipole/ferroelectric) will be coupled with the semiconductors, electrochemistry and/or photophysics which will not only solve the catalyst problem, but also develop a new field of science called ‘polarization photoelectrochemistry’, which IIT-Madras will be known for.
Under the Research Work Package (RWP), distorted materials will be developed and a clear correlation between polarization in these materials (oxides/halide perovskites) and the photo/electrocatalytic activity in solar water splitting and metal-air batteries will be investigated. In Phase I of the project at least 3 papers will be published in journals with Impact Factor great than 20 and at least another 3 in journals with Impact Factor greater than 10. The proposed high-end characterization facilities coupled with already existing infrastructure at PIs lab including Scanning Electrochemical Microscope, Ferroelectric Test Station, and Time Resolved Single Photon Counting, can enable us achieve this target.
A Technology Work Package (TWP) is proposed to develop mechanically rechargeable metal-air battery packs of 15 Ah and 12 V with less than 15 minutes complete recharging time. An electric two-/three-wheeler powered by in-house built batteries will be designed and developed. With the outcome of the technology work package (TWP), a company on battery manufacturing will be spun-out.
Although this project targets two selected problems in photo- and electro-chemical energy conversion & storage, in future, problems statements will be extended to other applications with research funding from GoI or industries.
Energy conversion and storage by photochemical (PC), electrochemical (EC) and photoelectrochemical (PEC) methods is an exciting and challenging area where many serious scientific questions remain. Answering these scientific questions can potentially help solving energy related problems. PC/EC/PEC devices have multiple applications in energy conversion and storage devices including solar water splitting,1 carbon dioxide reduction to fuels,2 nitrogen conversion to ammonia,3 metal-air batteries,4 fuel cells and electrolyzers.5 Most of these devices, although being under scientific investigation for several decades, have not seen a biggest commercial success. To make them into the commercial reality en-masse, both the fundamental principles of photoelectrochemistry and the materials associated with it need to be revisited, simultaneously employing widely available non-toxic and green resources.
Water is an important compound in the earth which is required for basic living as well as for many energy applications. The chemical reaction H2O↔H2+1/2O2 has multiple utility where, (i) solar energy can be stored in the form of chemical fuels by splitting water to generate hydrogen fuel and oxygen and (ii) the reverse reaction can be employed in metal-air batteries.4 The key to make commercial success with these devices lies in developing stable and highly effective catalyst that can carry out water oxidation to oxygen, oxygen reduction to water and water reduction to hydrogen. There are at least 270+, 300+ and 100+ articles in 10+, 20+ and 30+ impact factor journals respectively, in the area of water oxidation and oxygen reduction, between 2019 and 2020. These numbers highlights the importance of the research in this domain and their possible potential application in various energy conversion devices. Considering the necessity of these reactions, the C-PEC will work on novel research concepts in the area of photocatalysis, electrocatalysis and photoelectrocatalysis that can lead to very high impact publications. The target of the C-PEC will not be limited only to high impact papers, but also the problem statements are selected in such a way, that a new field of science/technology that IIT-Madras will be known for, is proposed. The new field will bring the domain of polarization to semiconductor photoelectrochemistry which will combine the conventional interdisciplinary research on semiconductors, photophysics and electrochemistry to ferroelectricity/dipole polarization. This flagship research domain will be an integral part of the fundamental research. Considering the sustainability of the Centre’s research activities, this fundamental concept on polarization photoelectrochemistry will be applied to achieve water oxidation in solar fuel production systems and oxygen reduction in metal-air batteries that will bring the commercial angle to the project.
Oxygen Evolution Reaction (OER) and Oxygen Reduction Reaction (ORR) are the limiting reactions in the aforementioned applications because of the sluggish four electron kinetics. So far several thousands of catalysts were developed focussing predominantly on tuning (i) electronic properties of the surface of the catalyst to increase the catalytic efficiency, (ii) bulk electronic properties to improve the conductivity for charge transfer, (iii) optical properties to enhance the visible sunlight absorption, and (iv) optoelectronic properties to improve the charge transfer kinetics. The effect of polarization induced by the material distortion is completely a un(der)explored area in any domain of catalysis.
The distortion in the material can potentially induce a local dipole in a centrosymmetric materials or a permanent polarization like in ferroelectric non-centrosymmetric materials. The perovskite materials, either with ABO3 or ABX3, structures can be easily tuned to obtained distortion. The halide perovskite are good photovoltaic materials, hence they will be employed in the PC/PEC, whereas oxide perovskites have good catalytic properties which will be employed for OER in EC. The crystal structure of the materials will be systematically distorted from completely symmetric to asymmetric systems, to induce varying degrees of polarization. OER and ORR reactions will be carried out to correlate the degrees of polarization to the catalytic ability. Once the concept is verified in perovskite materials for OER/ORR, it can be extended to any class of materials where polarization shall be induced and for any catalytic reaction.
As a part of C-PEC’s technology development programme, mechanically rechargeable metal-air batteries will be developed for their application in electric vehicles. The electrification of vehicles using lithium ion batteries is in progress, however, the non-availability of elemental reserves like lithium, cobalt and nickel required, in India, for such a large scale implementation is a downside for economy, wherein we will have to rely on foreign mine reserves for our entire transportation fleets. To avoid the situation of these elements becoming the new oil, and at the same time, utilize the in-house resources, this project identified the key battery technology that can become the next market leader in electric vehicles. The metal-air batteries specifically, based on aluminium and zinc, have specific energy comparable to or higher than the state-of-the-art li-ion batteries, along with incredible safety as the former devices employ water based electrolyte. These technologies however have technological pitfalls including the delivery of high power like lithium ion batteries, and low cycle life. Generally, metal-air batteries consist of metal anode, oxygen cathode, which is utilized directly from the atmosphere, gas diffusion electrode coated with oxygen reduction reaction (ORR)/ oxygen evolution reaction (OER) catalyst, and aqueous potassium hydroxide electrolyte. While discharging, the metal anodes react with oxygen to give metal oxide and electrons. The undesired reaction is a parasitic reaction of metals with aqueous alkaline electrolyte to give metal hydroxide species and hydrogen gas. This is also known as corrosion reaction, where the metal performance is degraded.
Basically, primary and secondary charging modes are adopted to evaluate the performance.
(i) In primary mode where the battery will be charged mechanically by replacing discharged metal with new metal anode, which takes only a few minutes. The discharged anode containing metals oxide can be recycled back to metals outside the battery in centralized locations using the well-established electro-winning process using electricity generated from renewable energy sources. Also, commercially available ORR catalysts will be utilized in the battery and the capacity will remain constant over its lifetime.
(ii) Secondary mode where the battery can be charged electrically, where metals oxide will split into active metals (Zn, Fe, Al, Mg) and oxygen within the battery. In this case, we need a bi-functional catalyst that can do ORR while discharging and OER during charging. The complete charging will take a few hours, which is not desirable for EVs. Also, there are some fundamental challenges like dendrite formation on metals surface while electrical charging, low performance, and bi-functional catalyst stability, which need to be solved before implementing in a large scale. Hence, we are focused on developing ultra-fast mechanically rechargeable metal-air (Al-air & Zn-air) batteries because of their favourable operating mechanism in EVs. These mechanically recharging systems will enable recharging the batteries in less than 15 minutes. The C-PEC’s plan is to develop complete mechanically rechargeable metal-air battery packs, couple it to the existing two-/three-wheeler vehicle designs and demonstrate a vehicle running in our IIT-Madras campus.
Overall, in this project, the Research Work Package will aim at developing a new field of science that couples the domain of polarization to semiconductor photoelectrochemistry which can potentially provide synergic effects in catalytic performances of OER/ORR for solar water splitting and metal-air batteries. The Technology Work Package will couple mature metal-air battery technology to the polarized ORR catalysts for electric vehicle battery pack development.Expected deliverables of the research
Phase 1:
High impact publications in journals with impact factors greater than 20 in the areas of
Polarization photoelectrochemistry applied to water splitting and metal-air batteries, that combines both experimental and simulation research.
Root cause analysis of various metal-air battery failure modes and providing solutions to avoid them.
Patents
- Fabrication techniques of gas diffusion layer and anodes for metal-air batteries
- Various compact designs of metal-air batteries and flow channels
- Devices for metal anode recycling and re-fabrication
- Material development for carbon dioxide adsorption from air
Prototypes
- Demonstration of small scale mechanically rechargeable metal-air battery design with fast recharging facility, which will power PMSM motor drive. Both will be connected through necessary power electronics developed in phase 1
Phase 2:
Deliver a new field science that will combine the polarization in the centrosymmetric or non-centrosymmetric materials to the photo-/electro-/photoelectro-catalytic properties. This will remain as a flagship research area of IIT-Madras, with publications in very high impact (30+ impact factor) journals.
Patents
- Metal air battery management system
- CO2 filtration unit design with material developed in phase 1
- Zinc recycling process
Prototypes
- Large scale metal air battery integrated with flow channels, CO2 filtration unit, battery management system and fast discharging it to drive PMSM motor. Estimation of overall system efficiency, reusability of zinc anode, battery life time in real conditions and operational costs.
- Electric two-/three-wheeler powered by metal-air battery
Start-up
- Deep tech start-up which is a leading metal air battery manufacturer for all types of electric vehicles and grid energy storage.
Current status
In order to indulge the polarized semiconducting materials with varying degree of distortion for Photocatalysis or Photoelectrocatalysis, herein a study has been proposed on three-dimensional (3D) and two-dimensional (2D) halide perovskite semiconductors. The distortion in these perovskites (varying bond lengths and bond angles) introduce significant optical properties making them an effective choice for various applications. This implies that due to applied electric field there can be a distortion through local and bulk polarization which can influence the charge separation in these systems.6
The invention of 3D halide perovskites with general formula ABX3 [where A = methylammonium (MA+), formamidinium (FA+), Cesium (Cs+), B = lead (Pb2+), X = chlorine (Cl-), bromine (Br-), iodine (I-)] gained immense popularity owing to its optoelectronic properties that revolutionized the field of photovoltaic and solar energy conversion in general.7,8,9,10 In search for an alternative to overcome the issues associated with lead-based perovskite, several strategies were adopted taking into consideration the Goldschmidt tolerance factor and band structure of lead-halide perovskite, and a new class of materials namely double halide perovskites11,12,13 were introduced with general formula A2BB’X6 showing comparable optoelectronic properties with an added advantage of better stability and less toxicity than the lead-based perovskites for diverse photocatalytic, photoelectrocatalytic and photoelectrochemical applications.
A lead-free halide perovskite with long carrier recombination lifetime14 will be effectively used as photo electrode for HER and OER reactions. Organic and inorganic metal-halide perovskites in present date are used for photocatalytic and photoelectrocatalytic applications with the help of surface passivation due to their instability in ambient atmosphere.15,16,17,18 Such passivation strategies can also be used for lead-free 3D perovskites to make them feasible for application in solar water splitting.19
Recently, 2D organic-inorganic perovskites have also attracted a great interest along with the 3D perovskites because of their improved material stability, excellent charge transportation and high degree of structural flexibility. Hydrophobic bulk aliphatic or aromatic organic amines are accommodated between the metal-halide layers via Vander-Waals force and induce heat (phonon) due to lattice distortion can offer ferroelectricity in these materials20,21. Compared to p-n junction type semiconductors, which usually require complicated interface engineering and fabrication processes, ferroelectrics present a nice alternative for generating a controllable power supply in single phase homogeneous materials by polarization22.
For practical applications in 2D ferroelectric materials, above the room temperature TC (Curie temperature) is needed to satisfy room and high temperature operating conditions reported for (BA)2PbCl423, (EA)2(MA)2Pb3Br1024, (BA)2(MA)2Pb3Br1025, (BA)2CsPb2Br726 and (2-Fluorobenzylammonium)2PbCl427 with notable spontaneous polarization and semiconducting properties. Although, progress have been made to resolve the environmental concerns like metal toxicity and led paths to design new lead-free polarized materials. Replacing the two divalent lead cations (Pb2+) with combined trivalent (In3+, Bi3+, Sb3+) and monovalent metals (Ag+, Na+) made a series of lead-free 2D halide double perovskites28,29 like (BA)4AgBiBr828 and (BA)2CsAgBiBr728. However, significant efforts are required to push the boundaries of ferroelectric design in these materials by understanding the structure-property relation.
However, ferroelectric halides perovskite exhibits good structural stability and appreciable optoelectronic properties, the implement of these materials towards PC/PEC applications is still in progress. This is because of the lack of scientific understanding especially in tuning materials between stability and performance. In addition, understanding the band structures phenomena of the ferroelectric perovskite halides is more important. This proposal aims to deliver the distorted ferroelectric perovskite halides with local and bulk polarization effects in PC/PEC and to understand the new field of science.
Though mechanically rechargeable metal-air batteries have high theoretical energy density and proven to be suitable for electric vehicles, they are still not implemented in large scale due to failure of battery earlier than the expected life time and inefficiency in utilising total energy stored. Companies like Yardney Electric Corporation and Electric Fuel Limited have attributed this premature failure of mechanically rechargeable metal air batteries to pore blocking in gas diffusion layer. Carbon dioxide in air reacts with highly alkaline electrolyte in battery at the air-electrolyte interface that exists on gas diffusion layer to form carbonate precipitates, thus blocking the pores and restricting the flow of oxygen reactant from air into battery which leads to failure. The metal air battery which is exposed to atmospheric carbon dioxide is failing after mechanically replacing metal cassettes ~400 times (approximately one year of operation). It is expected to increase the battery life time, if the air feed is free of CO2. Researchers are working on various technologies to filter carbon dioxide from the air feed to increase the battery life. Out of the widely known CO2 removal methods like adsorption, absorption, membrane and cryogenic gas cleaning techniques,30–32, adsorption has been considered to be a promising method to remove low concentrations of CO2 from air33,34. Amunátegui et al.,35 tested a pilot scale electrically rechargeable metals air flow battery where they used adsorption drying technology to remove carbon dioxide from ambient air. They achieved maximum of 2000 charge-discharge cycles (760 hours) in an accelerated durability test. The failure of battery after 2000 cycles occurred due to electrolyte flooding, which occurs due to erosion of hydrophobic layer coating. Though the removal of CO2 has increased life time of battery, the filtration equipment is too big to implement in an electric vehicle. Israel based company, Phinergy, claimed that they developed a compact gas diffusion layer structure that can block CO2 entering into the battery and hence increased the battery life to few thousands of hours. But the detailed technology is not revealed in public yet. Adsorption on high surface area specialised materials is gaining lot of attention these days. Shih-Cheng et al.,36 constructed a sorption-type air filter impregnated with MgO and CaO which adsorbed >95% of the inlet CO2 at low air flow rates. As flow rate increases, CO2 adsorption efficiency fell below 88%. The maximum surface area available in these materials for adsorption is found to be 957 m2/g. But the amount of CO2 adsorbed per unit mass of material is not specified, which is a critical parameter to understand the durability. Mehrdad Asgari et al.,37 developed sodalite-type metal organic frameworks with maximum surface area of 2050 m2/g. This material can selectively adsorb CO2 in very less time with high efficiency. Once this material is saturated with CO2, it can be slightly heated to desorb the CO2 and can be reused, thus increasing durability. The compact, lightweight, cheap and durable nature of metal organic frameworks makes this filter highly suitable for CO2 filtration in electric vehicles.
Though CO2 free air feed has potential to increase the life time of battery significantly, this alone is not sufficient to achieve competitive life time compared to that of lithium ion batteries. Other failure mechanisms like electrolyte leakage due to hydrophobic layer erosion35, power fading due to catalyst layer erosion or catalyst degradation, settling of negative electrode reaction products on positive electrode due to failure of separator will reduce the life time of battery. Researchers studied the degradation mechanism of Pt/C catalyst in various ways like methanol poisoning, agglomeration of active sites38,39. But root cause for other failure modes in metal air batteries is not very well understood. It is very important to study the performance of battery using in-situ and ex-situ analysis, operated in conditions those batteries undergo in an electric vehicle to get a collective insight into all failure modes.
From the global commercialization point of view, market value of metal-air batteries are about 438 Million USD and it is expected to increase around 842 Million USD by 2030. The market value for these vehicles is still expected to grow significantly in the near future, partly driven by the adoption of various environmental norms and emission regulations. The factors such as demand in high energy density storage solutions and global shift towards green emission are also the reason for such drastic increase in market value of metal-air batteries. Several companies such as Phinergy, MAL Research and Development Limited, and Log9 Materials are working on developing metal-air batteries for electric vehicles. Zinc8 Energy Solutions Inc. collaborated with Digital Energy Corporation to install a 100 kW/1.5 MWh zinc-air energy storage system in Brooklyn, New York, US40. Phinergy partnered with Indian Oil Corporation Limited (IOCL) to develop ultra-lightweight metal-air batteries for electric vehicles41. Log9 Materials from Bangalore raised USD 3.5 million as a part of its Series A round, led by Sequoia India’s scale-up program Surge and Exfinity Venture Partners LLP42. NantEnergy acquired Sharp Electronics Corporation’s Energy Systems and Services business that develops and delivers innovative energy management products for the US market. The acquisition includes the SmartStorage behind-the-meter energy storage system. The acquisition of Sharp’s energy systems business and the SmartStorage brand created a strong foothold for NantEnergy’s business in the US market.
Therefore, we propose an innovative battery technology called mechanically rechargeable metal-air battery for EVs. In this architecture, metal (Zn, Fe, Al) can be used as anode plates. The good performing anode with high energy density and utilization can be selected for fabricating large scale prototype devices. Simultaneously, a small amount of electrolyte can be circulated through the battery, which can avoid passivation and enable the complete recovery of discharged product from a battery. Metal alloying (Sn, Mg, Mn and In) can suppress corrosion and increase its shelf life. Although alloying with lead and mercury has proved to suppress corrosion, they are toxic metals. Research efforts will be made to find the lead and mercury-free metal elements. The customized and indigenously designed recycling process will be developed to convert discharged product back to active metal to reduce burden on metal mining and to keep EVs operating costs as low as possible.
Collaborations
International Collaborations
- Wendy Queen, Assistant Professor, Swiss Federal Institute of Technology (EPFL), Switzerland.link
- Marcel Schreier, Assistant Professor, University of Winconsin-Madison, USA.link
- Mojtaba Abdi-Jalebi, Assistant Professor, University College London, UK.link
Industrial collaborations
- EV and eMobility Solutions, Ashok Leyland, Chennai
External international site links for project
Societal impact
The key societal impact that this project may bring in is the increase in the utilization of green energy. The solar fuels portion of the research aims at generating green fuel using solar energy and water. Over the period of next 5 years, the solar water splitting device if made commercially viable will create a significant impact in the renewable energy sector. One of the domains where CSR funds can be made available is (i) testing and evaluation of solar water splitting systems, and (ii) solar powered transportation which can potentially reduce the pollution from vehicle sectors.
Indirectly, the project on metal-air batteries will also have a societal impact, where the reliance on fossil fuels will be reduced, leading to a decrease in the anthropogenic green house gas emissions to atmosphere.
Sustenance statement
The proposed centre encompasses two major areas of research on polarization electrochemistry for solar fuels and mechanical rechargeable metal-air batteries. The PI’s lab already attracted three projects on the related topics from DST-Nanomission (Ferroelectric Photo Electrochemical Water Splitting), IMPRINT with our industry partner Ashok Leyland (Development of a Prototype Metal Air Battery Driven Electrical Drive for a City Bus Duty) and Mission Innovation (Earth Abundant and Scalable Two Dimensional Catalysts for Selective Photo-electrochemical Solar Conversion of Carbon Dioxide to Methane/methanol). The TWP problem statements in this proposal are carefully framed so as to attract industry partners to keep the C-PEC sustainable.
PI and CoPI’s have good track record in attracting funding from various industries and this expertise will potentially help bringing in funds for the Centre. The presence of the state-of-the-art facilities as proposed in the Centre, along with the existing world class facilities like scanning electrochemical microscope, ferroelectric test station, full-vacuum ATR-FTIR spectrometer, UV-VIS-NIR absorption spectrometer and picosecond resolution – time resolved fluorescence spectrometer at PIs lab, will improve the visibility and output further to attract more funding.